Ultra-low Temperature Sterilization with Nitrogen Dioxide
NO2 Sterilization: 10-30°C. Vacuum Optional.TM
Noxilizer sells the proprietary Nitrogen Dioxide sterilization process. Noxilizer’s expert microbiology and material compatibility team is ready to partner to solve sterilization challenges through every phase of the process.

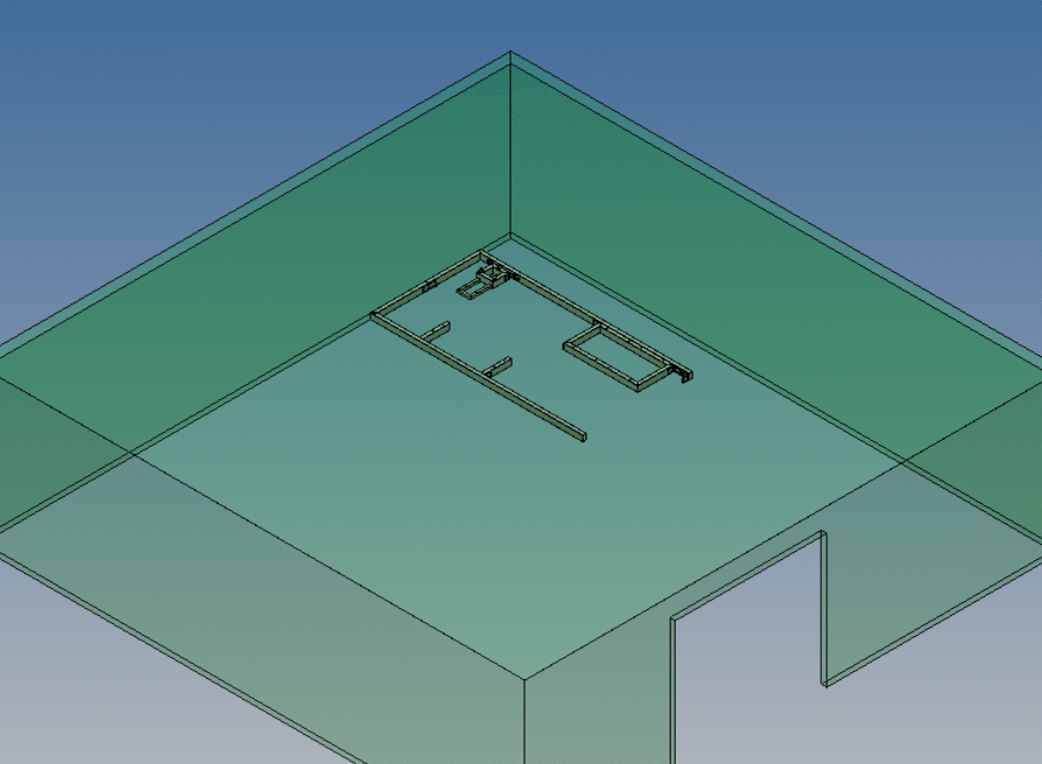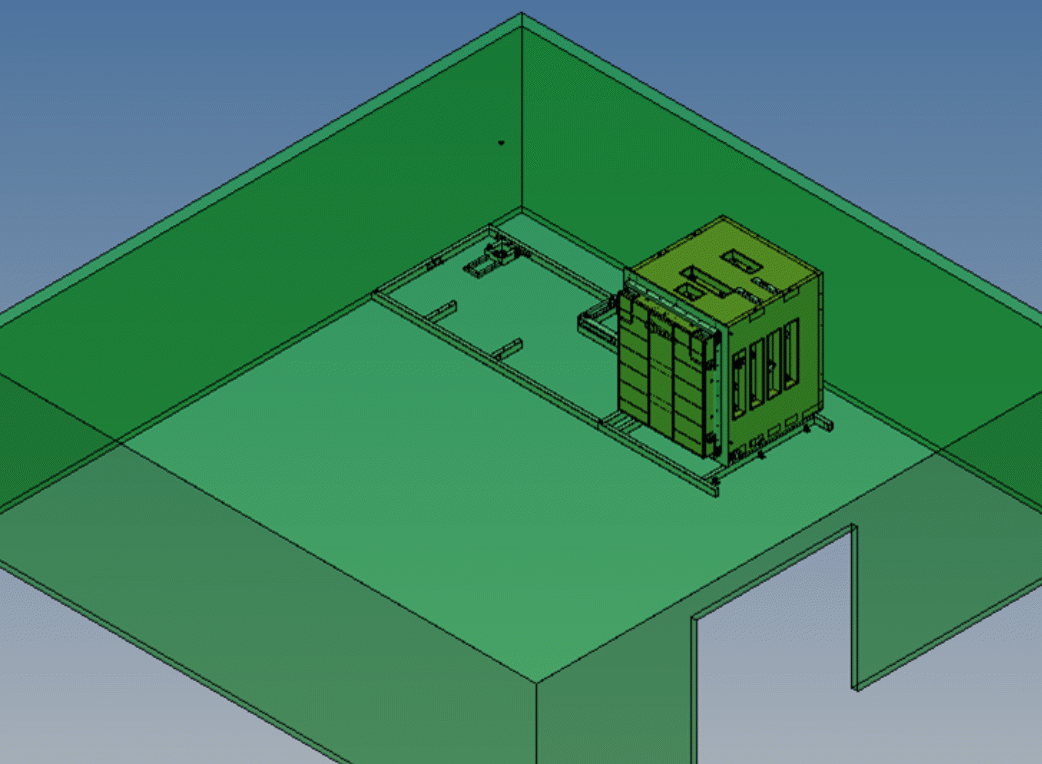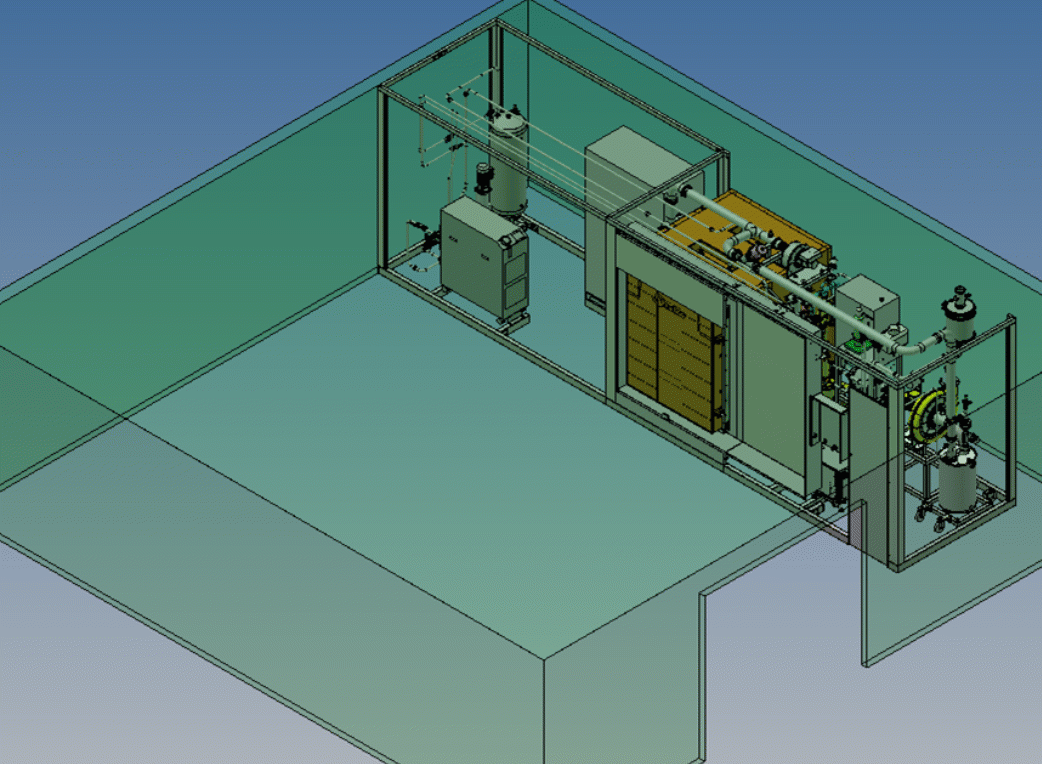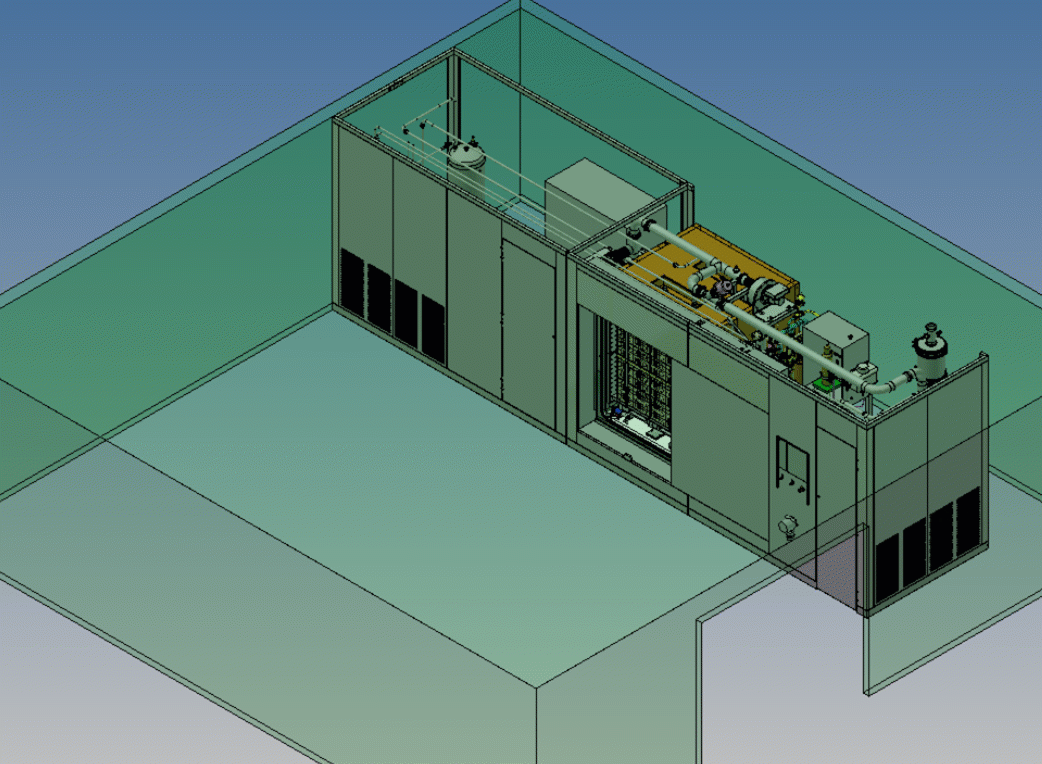
We provide ultra-low temperature (10°C to 30°C) nitrogen dioxide sterilization technology that offers many benefits over traditional sterilization methods.
Noxilizer is the expert in the application of the NO2 process within the supply chain.
Noxilizer sells sterilization equipment to pharmaceutical, biotech, and medical device manufacturers and also offers contract sterilization services.
Applications
Prefilled Syringes and
Combination Products
Nitrogen dioxide gas sterilization is ideal for these sensitive biotech products:
- Ultra-low temperature sterilization (10°C – 30°C) — maintains drug integrity
- Minimal vacuum option — prevents stopper movement
- Surface sterilization process — does not leach through the stopper
- Short cycle times (6-12 hours including aeration time)
- Safe and easy to bring sterilization in house — improves supply chain efficiency
Sensors
Nitrogen dioxide gas sterilization offers benefits for this new technology:
- Flexible humidity settings ranging from 40% to 85% — maintains sensor chemistry
- No cytotoxic residuals — protects sensor performance
- Safe and easy to bring sterilization in house — improves supply chain efficiency
Orthopedic Implants
Nitrogen dioxide gas sterilization reduces sterilization costs and accelerates turnaround time, especially for custom implants:
- Short cycle times (3-6 hours) — improves supply chain efficiency and significantly reduces sterilization costs
- Safe and easy to bring sterilization in house — returns timeline control to the medical device manufacturer
Recent Publications
Regeneron study notes that Nitrogen Dioxide (NO2) sterilization ensures drug integrity and lowers the risks of adverse effects with IVT
Posterior eye problems are often treated with intravitreal (IVT) therapies. However, IVT is linked with specific medication, equipment, and process issues that may lead to adverse effects. This study shows that by lowering the number of steps necessary for dosage preparation, pre-filled syringes can increase product stability and effectiveness and provide a safer alternative than glass containers when it comes to container closure and drug delivery. (Read More)

Terumo study finds that NO2 sterilized WFI-filled syringes have immeasurable NO2 ingress when compared to EO and VHP
Because of this, polymer-based prefilled syringes (P-PFSs) have revolutionized the pharmaceutical industry. However, to limit the risk of infection, exterior sterilization of P-PFSs is necessary. Though sterilization of P-PFSs has proven difficult with other technologies, Noxilizer’s sterilization technology uses NO₂ gas which effectively sterilizes the external surface of pre-filled syringes with no or minimal ingressing into the drug product — a technology that ensures drug integrity and lowers the risks of adverse effects with IVT. (Read More)
